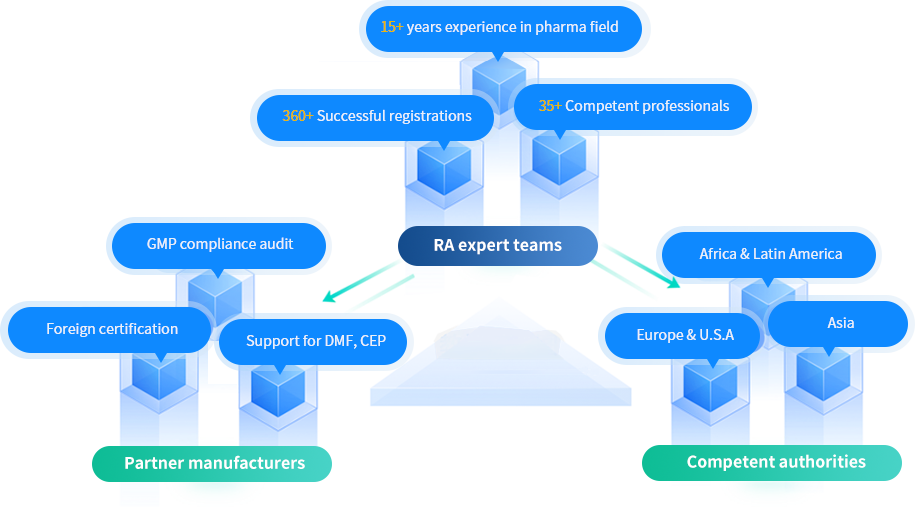
Full Range Regulatory and Compliance Services
Working closely with partner manufacturers, providing professional consultation for DMF registration and EU-GMP compliance, including DMF compilation, submission, and deficiency response, gap analysis, pre-inspection, improvement strategies, during the whole drug lifecycle, including annual report and post-approval change, to ensure drug registration approval and GMP compliance in the international markets, e.g. United States, Europe, Asia, Africa, Latin America, etc.
Reliable Communication Bridge between Manufacturer, Customer and Authorities
Establishing the reliable communication channel and bridge between the manufacturers, competent authorities and customers; Coordinating the technical discussions and providing the up-to-date guidelines and requirements to ensure all technical and regulatory queries are addressed and notified.